pH comprehensive guide: For measurement practice and applications
Parul Chhaparia, courtesy of Mettler Toledo | May 10, 2024Imagine a tiny, unseen key that unlocks the world's secrets, from the tang of a lemon to the health of our taste buds! It’s called pH! For various industries, including pharma, food, chemicals and the environment, pH, a seemingly simple measurement, has become a cornerstone for process control and quality assurance.
So, what exactly is pH, and why is it measured?
Simply put, pH stands for “potential of hydrogen.” It's a logarithmic scale used to specify the acidity or alkalinity of an aqueous solution. In other words, the scale determines the concentration of hydrogen ions (H+) in a solution. A pH of 7 means neutrality, while values below 7 are considered acidic, and those above 7 are deemed basic.
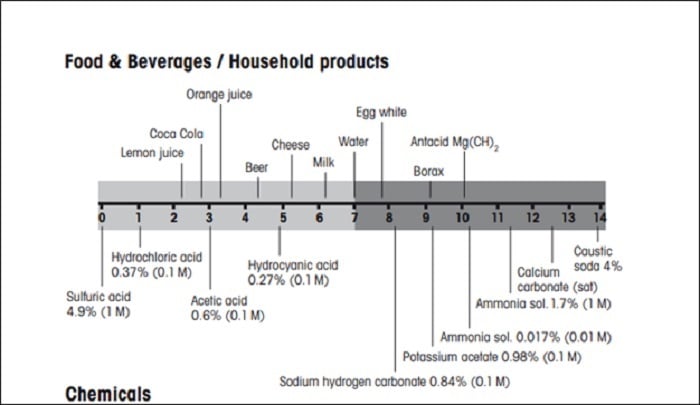 Figure 1: pH values of some chemicals and everyday products. Source: Mettler Toledo
Figure 1: pH values of some chemicals and everyday products. Source: Mettler Toledo
pH values have a significant impact on various industries. It is measured for different reasons. Some key examples are:
- To produce products with defined properties — during production, it is important to control the pH to ensure that the end product conforms with the desired specifications. The pH can dramatically alter the properties of an end-product such as appearance or taste.
- To lower production costs — this is related to the above-mentioned reason. If the yield of a certain production process is higher at a given pH, it follows that the costs of production are lower at this pH.
- To avoid doing harm to people, materials and the environment — some products can be harmful at a specific pH. We have to be careful not to release these products into the environment where they can harm people or damage equipment. To determine whether such a substance is dangerous we first have to measure its pH value.
- To fulfill regulatory requirements — as seen above, some products can be harmful. Governments, therefore, put regulatory requirements in place to protect the population from any harm caused by dangerous materials.
- To protect equipment — production equipment that comes into contact with reactants during the production process can be corroded by the reactants if the pH value is not within certain limits. Corrosion shortens the lifetime of the production line; therefore, monitoring pH values is important to protect the production line from unnecessary damage.
- For research and development — the pH value is also an important parameter for research purposes such as studying biochemical processes.
 Figure 2: pH product portfolio. Source: Mettler Toledo
Figure 2: pH product portfolio. Source: Mettler Toledo
These examples describe the importance of pH in a wide range of applications, demonstrating why it is so often determined.
The tools for pH measurements
The simple indication of pH values can be obtained using an indicator or pH paper, which changes to different colors depending on the pH level. However, industries require a much more precise practice of measuring pH. This is obtained with a pH measurement system that comprises a pH meter, a measuring electrode and a reference electrode.
A pH electrode consists of a pH-sensitive glass membrane and a reference electrode. When the electrode is immersed in a solution, the pH-sensitive glass membrane responds to changes in the hydrogen ion concentration of the solution. The reference electrode maintains a stable reference potential, allowing the pH meter to measure the potential difference between the two electrodes.
Reference electrodes
The reference electrode is an essential component of the pH electrode. It consists of a metal wire coated with a metal salt and immersed in an electrolyte solution. The metal salt coating generates a stable reference potential while the electrolyte solution maintains contact between the reference electrode and the measured solution.
Combination electrodes
Combination electrodes combine the pH electrode and reference electrode into a single unit. They are easier to use than separate electrodes, require less maintenance, and are less prone to contamination.
Beyond the basics
Besides the pH basics, there is much more to dive deeper into the pH measurement process and achieve maximum accuracy. Download our guide to get an in-depth understanding of:
• The science behind the pH scale
• Factors affecting pH measurements
• Selecting the right tool for your measurements
• Calibration techniques
To contact the author of this article, email pHmatters@mt.com