See a Close-Up of Lithium Dendrite Growth in Batteries
S. Himmelstein | October 28, 2017The microscopy technique that was awarded the 2017 Nobel Prize in Chemistry was used to document the first atomic-level images of dendrites, the finger-like growths responsible for short circuits or fires in batteries. Images were captured with cryo-electron microscopy, or cryo-EM, which images delicate, flash-frozen proteins and other “biological machines” in atomic detail.
The researchers observed that each lithium metal dendrite is a long six-sided crystal, not the irregular, pitted shape depicted in previous electron microscope shots. The ability to see this level of detail for the first time with cryo-EM will give scientists a powerful tool for understanding how batteries and their components work at the most fundamental level and for investigating why high-energy batteries used in laptops, cell phones, airplanes and electric cars sometimes fail.
“With cryo-EM, you can look at a material that’s fragile and chemically unstable and you can preserve its 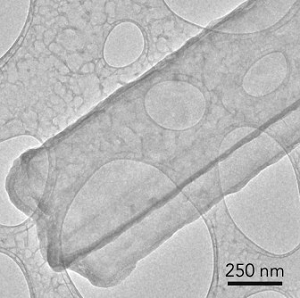 This image of a lithium metal dendrite, taken with cryo-EM, shows that freezing has preserved its original state, revealing that it’s a crystalline nanowire with six well-defined facets. (Credit: Y. Li et al., Science)pristine state – what it looks like in a real battery – and look at it under high resolution,” said Yi Cui, a professor at SLAC and Stanford. “This includes all kinds of battery materials. The lithium metal we studied here is just one example, but it’s an exciting and very challenging one.”
This image of a lithium metal dendrite, taken with cryo-EM, shows that freezing has preserved its original state, revealing that it’s a crystalline nanowire with six well-defined facets. (Credit: Y. Li et al., Science)pristine state – what it looks like in a real battery – and look at it under high resolution,” said Yi Cui, a professor at SLAC and Stanford. “This includes all kinds of battery materials. The lithium metal we studied here is just one example, but it’s an exciting and very challenging one.”
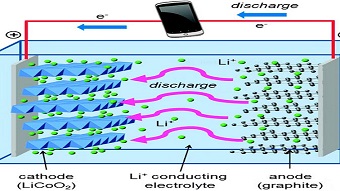
In cryo-EM, samples are flash-frozen by dipping them into liquid nitrogen and then sliced for examination under the microscope. A whole coin-cell battery can be frozen at a particular point in its charge-discharge cycle, allowing removal of a specific component and analysis of what is happening internally at an atom-by-atom scale.
This approach was used to examine thousands of lithium metal dendrites that had been exposed to various electrolytes. Metal parts of the dendrite were studied along with the solid electrolyte interphase (SEI) coating that develops as the dendrite reacts with the surrounding electrolyte. The coating also forms on metal electrodes as a battery charges and discharges, and controlling its growth and stability are crucial for efficient battery operation.
The dendrites were shown to be crystalline, faceted nanowires that prefer to grow in certain directions. Some of them developed kinks as they grew, but their crystal structure remained surprisingly intact in spite of the kinks.
A different method was applied to zoom in and examine the way electrons bounced off atoms in the dendrite, revealing the locations of individual atoms in both the crystal and its SEI coating. The atomic structure of the SEI coating became more orderly with the addition of a chemical commonly used to improve battery performance, a phenomenon which may help explain why the additive works.



The article says that some of them develop kinks. It would be interesting to know why some do and others do not and if that affects performance.